Project on atomic model and history:
Atom is the smallest constituent unit of ordinary matter that constituent of chemical elements viz electron, proton, neutron. Atoms are extremely small, typical sizes are around 100 picometers. In 1800 AD Jhon Dalton use the concept of atom to explain why chemical elements seemed to combine in ratios of small whole numbers. And Dalton give the atomic theory famous as "Dalton's atomic theory". Atom is mainly consist of two main part as nucleus and electron shell. The nucleus is made of proton and neutron. Proton is positively charged and neutron has no charge i.e neutral. And electron is negatively charge.
 |
| diagram of electron and proton |
Discovery of Electron:
In 1897, J J Thomson discovered that cathode rays are not electromagnetic waves but are made of particles taht are 1800 times lighter than hydrogen (the lightest atom). And that particle are electron and it is negatively charged. The symbol used for electron is  or
or 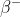 . The electron has charge value
. The electron has charge value  C. And mass is
C. And mass is  kg .
kg .
Discovery of Proton:
In 1886, Eugen Goldstein observed proton as 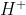 . In 1917 Ernest Rutherford identified and named Proton. Proton is positively charged paricle having charge opposit of electron i.e
. In 1917 Ernest Rutherford identified and named Proton. Proton is positively charged paricle having charge opposit of electron i.e  C. Mass of the proton is
C. Mass of the proton is  kg. Symbol used for proton p or
kg. Symbol used for proton p or  .
.
Discovery of Neutron:
In 1920, Ernest Rutherford theorized about neutron but after 12 year on 1932 his student James Chadwick discovered neutron. It is a neutral particle i.e have no charge . Mass of neutron is  kg. Most heavy particle in atom. Its is responsible for most of the mass of an atom.
kg. Most heavy particle in atom. Its is responsible for most of the mass of an atom.
Atomic model was first introduced by Jhon Dalton his statements were proved wrong. It does not matter that his model was not correct but he gave the first concept of atomic model and from his concept later more scientist developed atomic model.
Before Neils Bohr , Rutherford gave an atomic model but is was also need correction . According to Rutherford's nuclear model of the atom (it is a classical concept) : "atom is an electrically neutral sphere consisting of very small, massive, and positively charged nucleus at the cent surrounded by the revolving electron in their dynamically orbit".
But according to electromagnetic theory moving charge particle radiate electro magnetic radiation and that is nothing but and energy. So at some certain time the moving electron will loss his all energy and it will fall on the nucleus in a spiral way. Hence there will arise a question of existence of the atom. That's why this theory was declined by the scientist. Then Bhor Model gave the model of a stable atomic model.
Bohr's atomic model:
It is some time also called as Rutherford-Bohr model. In 1913 the model was prepared by Neils Bohr and Rutherford postulate of this model are as follows
1) The electron is able to revolve in certain stable orbits around the nucleus without radiating any energy, contrary to what classical electromagnetism suggests. These stable orbits are called stationary orbits and are attained at certain discrete distances from the nucleus. The electron cannot have any other orbit in between the discrete ones.

2)The stationary orbits are attained at distances for which the angular momentum of the revolving electron is an integral multiple of the Plank's constant: , where n = 1, 2, 3, ... is called the principle quantum number, and ħ = h/2π. The lowest value of n is 1; this gives a smallest possible orbital radius of 0.0529 nm known as the Bohr radius. Once an electron is in this lowest orbit, it can get no closer to the proton. Starting from the angular momentum quantum rule, Bohr was able to calculate the energies of the allowed orbit of the hydrogen atom and other hydrogen like atoms and ions. These orbits are associated with definite energies and are also called energy shells or energy levels. In these orbits, the electron's acceleration does not result in radiation and energy loss. The Bohr model of an atom was based upon Planck's quantum theory of radiation.
3)Electrons can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency ν determined by the energy difference of the levels according to the Plank relation: , where h is Plank's constant.











No comments:
Post a Comment